Goals
Below are the scientific goals of the network, which are covered in detail in this PDF. To overcome these challenges requires technological advances in imaging that the IBIN hopes to facilitate. If you can help us achieve these goals then sign up to our network here.
1 – Define the spatiotemporal dynamics of cell adhesion signalling
The process of molecular activation and unfolding events during cell-matrix adhesion complex formation is still not fully understood. Furthermore, how these signalling pathways are modulated by the extracellular environment is still to be fully elucidated.
It is the aim of the IBIN to develop 3D models and advanced microscopy techniques to further our understanding of these processes.
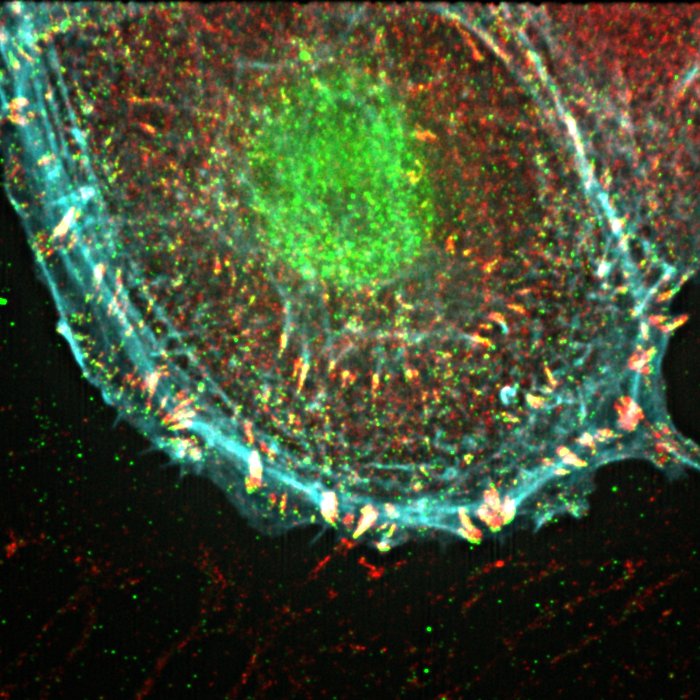
iSIM imaging of cell adhesion structures.
2 – Determine cell-specific cues that influence immune cell-tissue interactions
IBIN aims to develop probes that enable the determination of how altered signals from cells/tissue influence immune cell migration and interactions. Cancer cells in 3D ECM and monolayers of lung epithelial cells on 3D scaffolds will be co-cultured with immune cells.
Advanced imaging techniques will be used to determine the interactions between these cells/tissues and shed light on the interplay between the immune system and cancer.

3D spheroids forming in suspended drops.
3 – Determine how tissue mechanics influence cell growth and signalling
Define how the ECM controls cell behaviour over a timescale of minutes to hours. This will be via ECM organisation, mechano-sensitivity and cell phenotypes. 3D models, such as scaffolds of tuneable stiffness and mouse models will be used to identify the gene activation and signalling pathways regulated by the ECM.
Optogenetics and imaging techniques such as photoactivation will enable the identification of the role of ECM in cell growth and signalling.
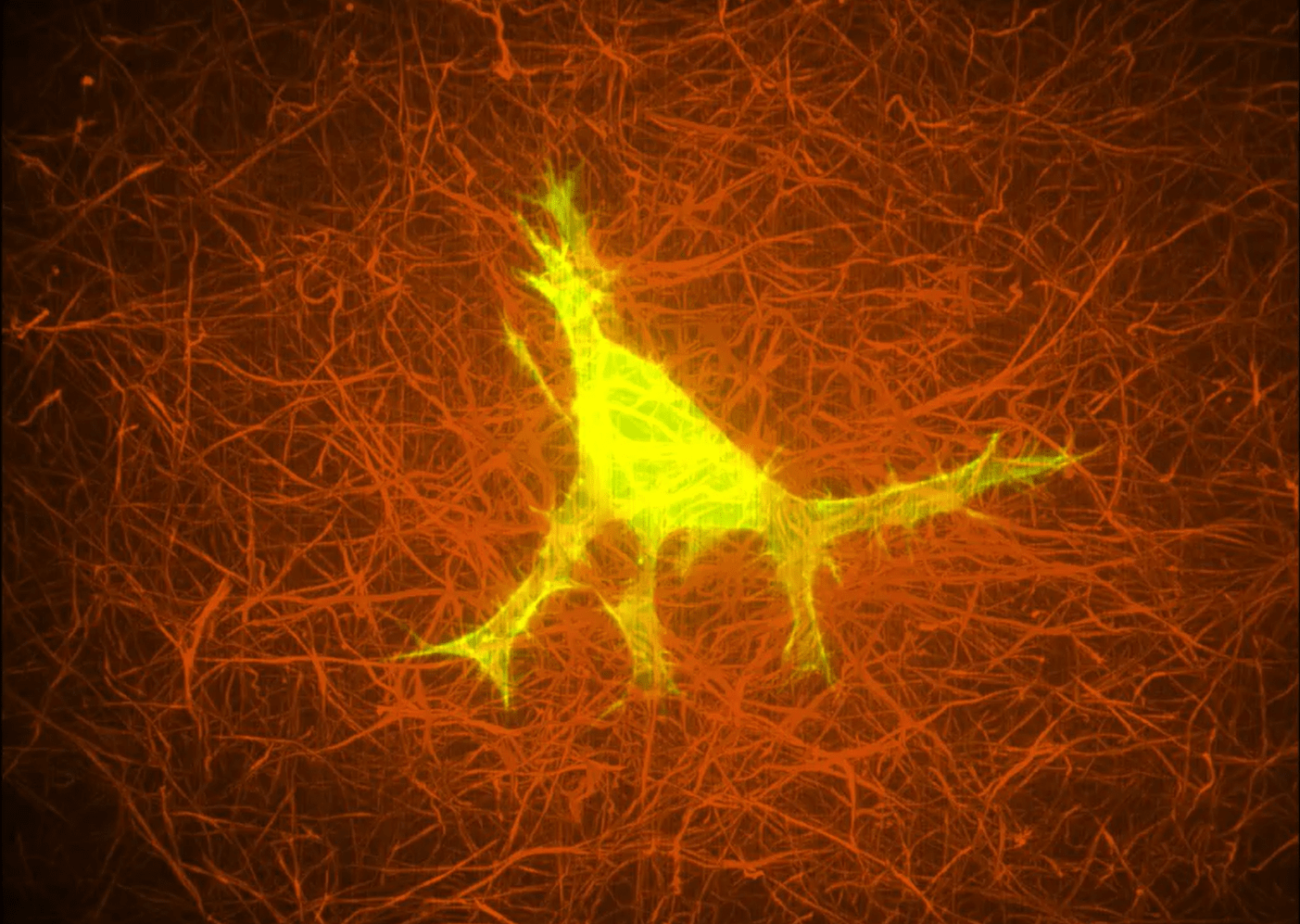
A cell embedded in a collagen matrix.
2 – Determine cell-specific cues that influence immune cell-tissue interactions
IBIN aims to develop probes that enable the determination of how altered signals from cells/tissue influence immune cell migration and interactions. Cancer cells in 3D ECM and monolayers of lung epithelial cells on 3D scaffolds will be co-cultured with immune cells.
Advanced imaging techniques will be used to determine the interactions between these cells/tissues and shed light on the interplay between the immune system and cancer.

3D spheroids forming in suspended drops.
3 – Determine how tissue mechanics influence cell growth and signalling
Define how the ECM controls cell behaviour over a timescale of minutes to hours. This will be via ECM organisation, mechano-sensitivity and cell phenotypes. 3D models, such as scaffolds of tuneable stiffness and mouse models will be used to identify the gene activation and signalling pathways regulated by the ECM.
Optogenetics and imaging techniques such as photoactivation will enable the identification of the role of ECM in cell growth and signalling.

A cell embedded in a collagen matrix.
3 – Determine how tissue mechanics influence cell growth and signalling
Define how the ECM controls cell behaviour over a timescale of minutes to hours. This will be via ECM organisation, mechano-sensitivity and cell phenotypes. 3D models, such as scaffolds of tuneable stiffness and mouse models will be used to identify the gene activation and signalling pathways regulated by the ECM.
Optogenetics and imaging techniques such as photoactivation will enable the identification of the role of ECM in cell growth and signalling.

A cell embedded in a collagen matrix.
